Product Detail

Basic Information
Cas: 17455-13-9
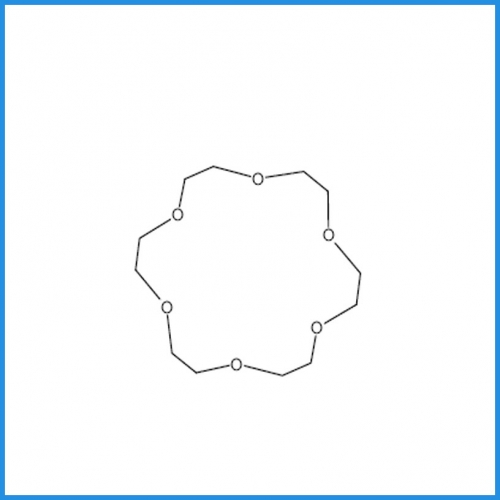
Name: 18-crown-6
1,4,7,10,13,16-Hexaoxacyclooctadecane;[18]crown-6;18-Crown-6;18-Crown 6-Ether;18-Crown ether-6;CROWN-18-5- ETHER;
Molecular formula: C12H24O6
Molecular weight: 264.31500
PSA: 55.38000
LOGP: 0.09960
Physical index
Appearance and properties: colorless crystal
Density: 1,175 g/cm3
Boiling point: 116°C 0,2mm
Melting point: 42-45 °C(lit.)
Flash point: >230 °F
Refractive index: 1.4577 (50ºC)
Water solubility: SOLUBLE
Stability: Stable. Incompatible with strong acids, strong oxidizing agents.
Storage conditions: Store at 0-5ºC
Vapor pressure: 4.09E-06mmHg at 25°C
Security Information
RTECS number: MP4500000
Safety instructions: S26-S36-S39
WGK Germany: 3
Hazard category code: R20/22; R36
Customs code: 2932999099
Dangerous goods transport code: 2811
Hazard category: 6.1(b)
Packing level: III
Dangerous goods mark: Xn
Signal word: Warning
Hazard description: H302
Danger sign: GHS07
Hazard prevention statement: P301 + P312 + P330
Production methods and applicationproduction method
1.It is usually prepared by the William Lin synthesis method, that is, the reaction of alkoxide and alkyl halide.
2. Make bis(2-chloroethyl) ether in a 1-liter three-necked flask equipped with a stirrer, reflux condenser, thermometer and dropping funnel, place 63.7 g (0.6 mol) diethylene glycol, 400 ml benzene, 102.8 .G (1.3 mol) of adixa, 155 g (1.3 mol) of sulfite chloride was added dropwise within 1.5 hours while stirring at 75°C. Continue to reflux for 12 hours, acidify with hydrochloric acid to PH=2, separate the organic phase, and dry with anhydrous sodium sulfate. Filter, evaporate the solvent and distill under reduced pressure to collect the 50-52℃/5-8mm fraction to obtain 76 grams (86%) of bis(2-chloroethyl) ether,
3. 2. Make 18 a crown and a 6 device Same as above. In a 3-liter three-necked flask, place 416 grams (about 6.3 moles) of 85% granular potassium hydroxide, 243 grams (1.25 moles) of tetraethylene glycol, and 1 liter of tetrahydrofuran. After heating slowly for 15 minutes, under vigorous stirring A solution of 447 g (3.125 mol) of bis(2-chloroethyl) ether and 150 ml of tetrahydrofuran was added. Continue to reflux for 18 hours under stirring. The liquid was distilled off under reduced pressure, and the remaining brown slurry was diluted with 750 ml of dichloromethane. Filter and wash the solid salt with 100 ml of dichloromethane. Combine the filter solution and lotion, add magnesium sulfate to dry. Filter, concentrate under reduced pressure, and then distill under reduced pressure in the presence of nitrogen to collect the fraction of 110-120/0.2mm.
(l). Preparation of 1,8-dichloro-3,6-dioxa-octane in addition to three Except that glycol replaces diethylene glycol, the rest is the same as the preparation of bis(2-chloroethyl) ether.
(2). The system 18-Crown-1 6 device is the same as above. In a 3-liter three-necked flask, place 112.5 g (102 ml, 0.75 mol) of triethylene glycol and 600 ml of tetrahydrofuran, and add 60% potassium hydroxide solution under stirring (the 109 g of 85% (1.65 mol) Granular potassium hydroxide is dissolved in 70 ml of water.'' After slowly heating and stirring vigorously for 15 minutes, the solution began to change color and gradually formed a dark brown. Add 140.3 g (0.75 mol) 1,8-dichloro-3,6-dioxane A solution of octane in 100 ml of tetrahydrofuran is refluxed under vigorous stirring for 18-24 hours. Cool, evaporate most of the solvent under reduced pressure, and then add 500 ml of dichloromethane to dilute the remaining brown slurry. Filter with a sand core funnel, wash with dichloromethane, combine the filtrate and lotion, and add anhydrous magnesium sulfate to dry .
Filtration, high vacuum distillation after concentration under reduced pressure, collect 100-167℃/0.2mrn fraction, the main fraction contains 76-78 g (38-44%) 18-crown-6 crude product. Through careful distillation and recrystallization (or liters, or chromatography), purified products can be obtained. Purify in a 250 ml Erlenmeyer flask, place 50 g of 18-crown-6 crude product, 100 ml of ethyl alcohol and a magnetic stirring rod, and then install calcium chloride in the drying tube. The mixture was stirred into a slurry, heated on a hot plate to dissolve, and then cooled to room temperature under vigorous stirring, allowing fine white crystals (crown ether-ethyl umbilical complex) to settle. After placing it in the refrigerator for 24 to 28 hours, let it stand in a cold bath at 30°C to make the complex precipitate as complete as possible.
Quickly filter in an ammonia-filled drying box or a nitrogen-filled funnel, wash the hygroscopic crystals with a small amount of cold acetonitrile, and then transfer to a 200 ml round-bottomed flask equipped with a gas tube with a piston and a magnetic stir bar, and the temperature is 0.1-0.5 Warm in a mm vacuum
use
Complexing reagents and phase transfer reagents.

 English
English français
français русский
русский español
español العربية
العربية