Bubble control is necessary in many industries, such as papermaking, water treatment, pharmaceuticals, dyes and coatings.With the continuous upgrading of national environmental protection regulations and the continuous improvement of residents' environmental awareness, water-based coatings have made great progress.Emulsifying agent and wetting dispersant in waterborne coating formula reduce the surface tension of the system and stabilize the bubbles in the system easily.The existence of bubbles will have a negative effect on the production and coating of coatings.During the grinding process of paint, the "air bag" formed by bubbles around the filler reduces the transfer efficiency of shear force and increases the grinding time.After coating, the residual dry foam on the surface will not only affect the appearance of the coating, but also become the center of corrosion, reducing the durability of the coating.In order to eliminate these problems, almost all waterborne coatings need to add defoaming agent.The active component of defoaming agent can achieve the purpose of defoaming by interfering with and destroying the stabilizing effect of bubbles.Different types of defoaming agents act in different ways, so understanding the mechanism of action of defoaming agents can help to systematically adjust the key physical and chemical parameters, so that the main steps in the mechanism can be controlled to achieve the best defoaming efficiency.
1, the stability of bubbles
Water - based coatings in the production process, mechanical mixing is easy to bring air into the system.In the process of coating construction, brush, roller and spray are easy to bring gas into the wet film.Other porous substrates, such as wood panels and cement, release gases into the film as the paint wets and penetrates.The gas brought into the coating system is surrounded by the liquid phase and forms a bubble, and relies on electrostatic action and surface tension gradient to maintain stability.
1.1 Electrostatic Action
Water-based coating formula contains a variety of surfactant substances, the special point is that the molecule contains a polar or charged hydrophilic end base and a hydrophobic hydrocarbon chain.This unique molecular structure makes it easy for surface activators to form micelles in a directional arrangement at the gas-liquid interface, as shown in Figure 1, with the hydrophilic end facing the water phase and the hydrophobic end facing the air.
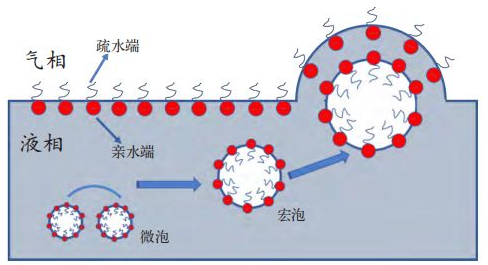
Because the density of air is less than the paint, so once the bubble will rise to the paint interface.According to Stokes' law, the bubble rising rate depends on the radius of the bubble and the viscosity of the paint [4].The larger the half diameter of the bubble, the lower the viscosity of the coating, the faster the bubble rise.Microbubbles usually exist in coatings with large specific surface area, resulting in high surface freedom energy.Thermodynamically, microbubbles are so unstable that they spontaneously fuse with each other to form bubbles with smaller specific surface areas and thus lower free energy.When the macrobubble rises to the liquid level, the hydrophilic group at the gas-liquid interface on the bubble and the hydrophilic group at the coating's gas-liquid interface produce mutually repulsive effect, so that the bubble is in a stable state and not easy to crack.
1.2 Marangoni effect
When there is no surfactant in pure water system, the bubbles produced by external force rise from the liquid body to the liquid level.Due to the action of gravity, the liquid at the top of the bubble film will flow back to the liquid body along the gas-liquid interface on the bubble film, resulting in a gradual decrease in the thickness of the bubble liquid film.When the liquid film thickness is less than the critical film thickness of 10 nm, the bubble will burst.In the presence of surfactants, as shown in FIG. 2(a) and FIG. 2(b), the surfactant molecules at the top of the bubble will also decrease as the liquid is discharged, resulting in a higher surface tension at the top than on the two sides of the bubble.Surface tension is an energy state that always tends to flow from low to high surface tension.In this way, the liquid on either side of the bubble will flow back to the upper part of the surface with high tensile force, producing a force contrary to the gravitational drainage, as shown in Figure 2(c). This reverse flow of liquid is called the Malangani flow.When these two forces reach an equilibrium state before the critical thickness of the bubble is reached, the stable bubble in FIG. 2(d) will be produced.
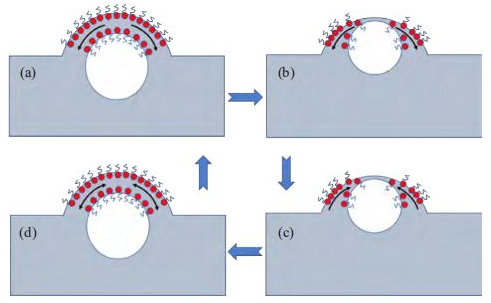
In this two-way flow process, the bubble film will take place a certain stretching process.If the bubble has certain elasticity, it is more conducive to the stability of the bubble.According to the Gibbs elasticity theory shown in Equation (1), where F is the elastic parameter, A is the bubble surface area, and γ is the liquid phase surface tension.In order for the bubble to obtain some elasticity, the surface tension of the liquid needs to change with the surface area of the bubble, so that the value of D γ/dA is greater than 0.
If the bubble cannot change its surface tension during the process of contraction and extension, the bubble will rupture due to higher rigidity.The surface tension of pure water hardly changes, so bubbles are not stable.
2. Mechanism of action of defoamer
Defoaming agent is a kind of auxiliary agent that destroys the double electric layer of surfactant around the bubble, making the bubble wall unstable and burst, thus releasing the coated gas.Typical defoamer mainly consists of carrier oil, active solid granules and emulsifier.Among them, carrier oil can be mineral oil, silicone oil, natural oil, white oil, etc., which can quickly transport active solid particles such as hydrophobic silica, paraffin or metal soap to the bubble film to play a role.Emulsifiers are used to regulate the compatibility of defoaming agents with the main phase of coatings.The principle of choosing the best defoaming agent is to find a balance between its defoaming efficiency and its compatibility with the system.Highly incompatible defoamer can act efficiently in the system, but because they cannot be fused into the system and migrate to the gas-liquid interface, it is very easy to produce surface defects.However, highly compatible defoaming agents quickly integrate into the coating system and are not sufficient to provide high defoaming efficiency.For the role of defoaming agent in waterborne coatings
System, can be divided into bridge - to wetting, bridge - stretching and fluid spread clamp 3 categories.No matter how the defoaming agent acts, the first thing is that the defoaming agent can enter the bubble film thin layer. Thermodynamically, the permeability coefficient E is used to express the difficulty of the defoaming agent entering the bubble film, which is expressed as Equation (2) :
γAW, γOW and γOA respectively represent the surface tension of the liquid, the interfacial tension of the liquid and the defoamer, and the surface tension of the defoamer.When E > 0, it indicates that the defoaming agent can enter the bubble thin layer and connect with its bilayer membrane to form a bridge, and the stability of the bridge effect also affects the action efficiency of the defoaming agent molecule, which is expressed by the bridge coefficient B in thermodynamics, and its expression is shown in Equation (3) :
Unstable, and can further play the role of defoaming.When B < 0, it forms a stable bridge, and the bubble is not easy to burst when it reaches the stability.When E < 0, it indicates that the defoamer cannot enter the bubble bilayer, but is excluded to the adjacent Platonic channel [9].Only when the capillary pressure gradually increases due to the gravity drainage of bubbles, and the Platonic channel film becomes narrow, can the defoamer be forced to enter the bubble film for spreading. At this time, the efficiency of the defoamer is closely related to the spreading coefficient S of carrier oil, which can be expressed as formula (4) :
S = γAW -γOW -γOA (4)
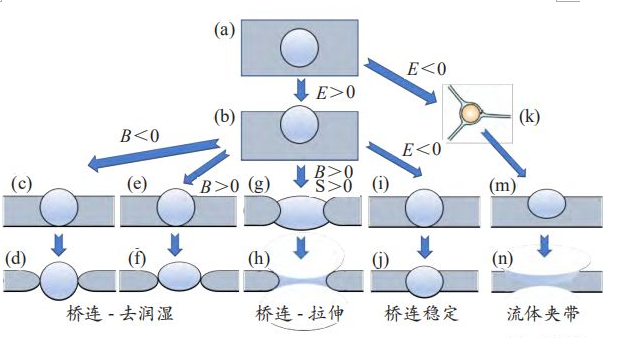
The results show that the antifoaming efficiency of carrier oil with higher spreading coefficient S is significantly higher than that of non-spreading carrier oil antifoaming agent.Therefore, permeability coefficient E, bridge number B and spread coefficient S play a decisive role in the process of defoiling.FIG. 3 shows the action mechanism of defoamer under different conditions.
2.1 Bridging - de-wetting action
When the permeability coefficient E of the defoamer is greater than 0, as shown in Figure 3(a) and Figure 3(b), the defoamer enters the bubble film, and when the bridging coefficient B is greater than 0, the active solid particles in the defoamer form a bridge with the bubble bilayer, and the liquid on the film layer is dewetting due to the strong hydrophobicity of the solid particle surface.As shown in Figure 3(c) and Figure 3(d), the bubble membrane was punctured and the bubble burst.Similarly, defoamer carrier oils with hydrophobic surfaces also have a dewetting effect.Different from solid particles, oil droplets have the ability to decompose and deform. When entering the bubble film, the defoaming agent oil droplets are deformed into prismatic shape and no obvious deformation occurs any more, as shown in FIG. 3(e) and FIG. 3(f). At this time, dewetting occurs.When the carrier oil and hydrophobic solid particles are in the same component of the defoamer, the synergistic effect makes the defoamer more efficient. This is because the presence of solid particles makes the bubble film puncture effect stronger, that is, with higher permeability coefficient, it is easier for oil droplets to enter the bubble film layer to play a role.
2.2 Bridging and stretching
When the oil droplet enters the bubble film layer and forms a bridging effect, when the spreading coefficient S of carrier oil is greater than 0, the spreading diffusion of oil droplet leads to the formation of oil droplet surface with different curvature at the oil-water interface and gas-water interface, as shown in FIG. 3(g).At this point, due to the action of non-equilibrium capillary pressure, the oil droplets gradually stretch and thin, as shown in FIG. 3(h), until they break, resulting in the rupture of the bubble.Silicone defoamer benefits from the high spreading coefficient of silicone oil and acts mainly through the bridging - stretching mechanism.When the bridging coefficient B < 0, stable bubbles in FIG. 3(I) and FIG. 3(j) will be formed.
2.3 Fluid entrainment
As mentioned above, when the permeability coefficient B < 0, the defoamizer is repelled to the Platonic channel adjacent to the bubble film, as shown in FIG. 3(k), and enters the bubble film under the action of non-equilibrium capillary pressure, as shown in FIG. 3(m) and FIG. 3(n).When the defoamer molecule reaches the second layer of the bubble bilayer, the surfactant is gradually replaced by adsorption due to its strong spreading ability.With the occurrence of malangani fluid movement, the carrier oil entrains the bubble film, which leads to the thinning of local bubble film and the final rupture.The prerequisite for the fluid entrainment mechanism is that the carrier oil has a good spreading ability, that is, S > 0.Some defoaming agents which do not contain hydrophobic solid particles mostly rely on this mechanism.
3 Classification of defoaming agent
Among them, mineral oil as the carrier of defoamer, mainly aromatic or aliphatic mineral oil, and aromatic mineral oil is easy to cause the risk of yellowing paint, and harmful to human physiology, has been rarely used.Hydrophobic particles are mainly silica, paraffin, metal soap or polyurea.A small amount of emulsifier in the defoamer can disperse the hydrophobic particles well in the carrier oil and improve the compatibility of the defoamer with the system.Due to environmental and health concerns, traditional APEO emulsifiers have been replaced by linear or branched fatty alcohol compounds.Mineral oil defoamer is mainly used in matte and semi - gloss emulsioni paint.For high quality water-based industrial coatings, the introduction of mineral oil defoaming agents can easily cause the risk of oil separation and gloss reduction on the surface.The main action mechanism of mineral oil dispersants is fluid entrain.
3.2 Silicone antifoaming agent
Silicone oil as a carrier of defoaming agent, the main components of polysiloxane or polysiloxane.The Si -- O bonds in polysiloxane polymers are quite flexible, and the silicon oxygen skeleton provides a high spreading coefficient, while the methyl group provides hydrophobicity and low surface tension.These properties make polysiloxane defoamer very effective.And polysiloxane can also be modified to improve its compatibility, such as the use of polyether chain to modify can improve the hydrophilicity of polysiloxane, so improve its compatibility in the polar system.Because it contains silicone, this type of defoamer is more expensive than mineral oils and is commonly used in high-end coatings.Silicone defoaming agent can also be combined with hydrophobic particles such as polyurea and silica to improve the dispersion and defoaming performance of silicone oil.Compared with mineral oil, the main advantage of silicone defoamer is that it will not lead to the gloss reduction of the high light system, nor will it affect the compatibility of the color paste of the system.The chemical structure of polysiloxane makes it a better surface tension reducer than non-silicon defoamer, which is useful because of its better permeability and spread coefficients.The main action of silicone defoamer is the bridging - stretching mechanism.
3.3 Molecular defoaming agent
Molecular defoamer is essentially a kind of non-ionic surfactants, which can compete for adsorption and substitution with the foam stable surfactants on the thin layer of bubbles.The wetting dispersant and emulsifier in the coating formula mainly stabilize the bubbles through the interionic force, hydrogen bond and van der Waals force, while the sub-defoaming agent destroys these forces at the molecular level to achieve the defoaming effect.Conventional defoaming agents are mainly through the incompatibility with the system to achieve the performance of defoaming, but it will also produce some side effects, such as surface defects, poor recoating and performance degradation after storage.As a surface active agent, molecular level defoamer is compatible with most systems and can provide wetting effect.In general, molecular defoamer achieves a good balance in defoaming efficiency and compatibility, and is very efficient for controlling both microbubbles and macrobubbles.Molecular defoamer is suitable for low viscosity, high gloss and low PVC paint and varnish.
Development direction of waterborne paint defoaming agent
With the development of water-based coatings, the demand for efficient defoaming agent is increasing.The research of defoaming agent in China has a history of nearly 20 years.The first generation of antifoaming agent is mainly based on animal and vegetable oil as the carrier.In the 1960s, the second generation of antifoaming agents based on polyether chains of epoxane blocks were born.The representative of the third generation of defoaming agent is polydimethylsiloxane as the main active substance, which is also the most widely used defoaming agent at present.
Based on the current situation of the application of defoamer in the coating industry, the future development of defoamer will mainly focus on the following four aspects:
(1) Improve the mechanical stability and storage stability of the existing active components to ensure high efficiency and durability in the coating system.Compatibility and defoaming efficiency can be balanced by chemical modification of existing active components or exploration of new active components.
(2) For different coating systems, develop special types of defoamer, from the traditional general type to the development of special type, maximize the function of customized defoamer.
(3) Instead of the traditional single component, poor economic benefits of the product, the development of complex synergy type of high efficiency defoamer products.
(4) From the perspective of environmental resources, the development of multi-functional new molecular level defoamer, such as both wetting function, reduce the minimum film formation temperature of coatings, reduce or discard the use of film forming additives, reduce VOC emissions.
As an essential additive in waterborne coatings, the efficiency of defoaming agent is not only affected by the other components in the coating formula, but also by the physical and chemical properties of the components of the defoaming agent itself, such as surface hydrophilicity, interfacial tension, rheological properties, etc.A deep understanding of the mechanism of action of defoamer is beneficial to the selection of the best solution of defoamer in practical application and lays a theoretical foundation for the development of new efficient defoamer.Benefit from the vigorous development of waterborne coatings, defoaming agent market prospects will be more broad.

 English
English français
français русский
русский español
español العربية
العربية







